
Here, we have synthesized Ca-Pv from different starting materials with varying H 2O contents at 19–120 GPa and 1400–2200 K in laser-heated diamond-anvil cell. However, possible hydrogen storage in the third most abundant mineral in the region, CaSiO 3 perovskite (Ca-Pv), is not well unknown. The lower mantle is believed to contain much less hydrogen (or H 2O) because of the low storage capacity of the dominant mineral phases, such as bridgmanite and ferropericlase. Based on these measurements, we report three principal findings: (1) pressure-induced changes in hydrogen bonding (proton disordering or hydrogen bond symmetrization) occur at substantially lower pressures in ε-FeOOH than previously reported and are unlikely to be linked to the high-spin to low-spin transition (2) ε-FeOOH undergoes a 10% volume collapse coincident with an isostructural Pnnm → Pnnm transition at approximately 45 GPa and (3) a pressure-induced band gap reduction is observed in FeOOH at pressures consistent with the previously reported spin transition (40 to 50 GPa). In this paper, the high-pressure evolution of FeOOH was re-evaluated up to ~75 GPa using a combination of synchrotron-based X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), and optical absorption spectroscopy. Additionally, the pyrite-type polymorph (space group Pa3¯ of FeOOH), and its potential dehydration have been linked to phenomena more » as diverse as the introduction of hydrogen into the outer core, the formation of ultralow-velocity zones (ULVZs), and the Great Oxidation Event. These structures potentially form a hydrogen-bearing solid solution with AlOOH and phase H (MgSiO 4H 2) that may transport water (OH –) deep into the Earth's lower mantle. Recently, a great deal of attention has been paid to the high-pressure polymorphs of FeOOH (space groups P2 1nm and Pnnm). Keck Foundation National Science Foundation (NSF), Directorate for Geosciences Division of Earth Sciences (GEO/EAR) National Aeronautics and Space Administration (NASA) OSTI Identifier: 1864759 Alternate Identifier(s): OSTI ID: 1657647 Grant/Contract Number: AC02-06CH11357 EAR1321976 EAR1401270 80NSSC18K0353 EAR-1128799 NRF-2018R1A3B1052042 FG02-94ER14466 Resource Type: Journal Article: Accepted Manuscript Journal Name: Earth and Planetary Science Letters Additional Journal Information: Journal Volume: 550 Journal ID: ISSN 0012-821X Publisher: Elsevier Country of Publication: United States Language: English Subject: 58 GEOSCIENCES crystal structure hydrogen hydrous iron oxide lower = ,Ĭonstraining the accommodation, distribution, and circulation of hydrogen in the Earth's interior is vital to our broader understanding of the deep Earth due to the significant influence of hydrogen on the material and rheological properties of minerals. (ANL), Argonne, IL (United States) Sponsoring Org.: USDOE W.M. Publication Date: Thu Sep 03 00:00: Research Org.: Argonne National Lab. Arizona State Univ., Tempe, AZ (United States).of Geosciences, Hubei (China) Arizona State Univ., Tempe, AZ (United States) As a result, the large channel in the crystal structure of the η phase could provide potential storage sites for other volatile elements in the deep mantle. Because of its limited H 2O storage capacity, which is less than 1/6 of the storage capacity of the pyrite-type phase and the ε phase, the stability of the η phase would result in H 2O loss during water transport in more » the mid mantle and therefore limit the amount of H 2O potentially stored in the Fe–O–H system of the lower mantle. Our experiment also shows that the new η phase can exist together with the major lower mantle minerals including bridgmanite and periclase, making it an important hydrogen-bearing phase in the Earth's deep interior.
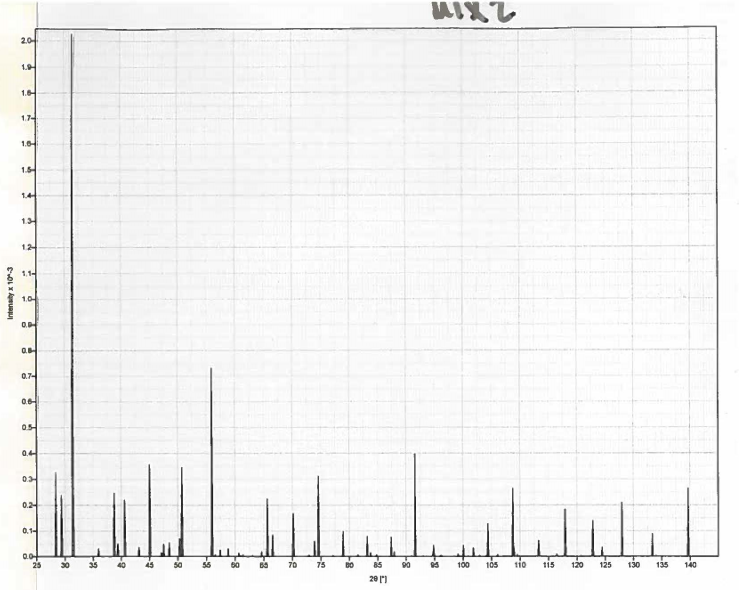
Here, we report a new hydrous iron oxide (η-Fe 12O 18+x/ 2H x, x ≈ 2) stable at pressures between the stability fields of the ε- and the pyrite-type FeOOH. However, it remains uncertain how the potential deep hydrogen storage can be connected to the shallower storage. These discoveries suggest possible hydrogen storage in the mantle transition zone and in the mantle below 1800 km depths, respectively. Pyrite-type FeOOH was found to be stable at pressures higher than 80 GPa. Recent experiments found titanium bearing ε-FeOOH in a hydrous basaltic system at 12–19 GPa and 1300 K. The amount of hydrogen stored in the Earth's interior is important for a range of issues, including the volatile incorporation during the Earth formation and the co-evolution of the atmosphere, the hydrosphere, and the interior.


 0 kommentar(er)
0 kommentar(er)
